TechneLite® Tc 99m Generator Supply Update
November 2022 - We would like to provide you with an update on the European reactor outage, the associated Moly supply disruptions, and the impact on our Xenon-133 production.
According to a memo on November 18 from Nuclear Medicine Europe, the Belgian reactor BR2 has cancelled its November cycle entirely. The Czech reactor LVR15 did successfully restart on November 18, and HFR in the Netherlands is expected to restart soon. Based on this information, Nuclear Medicine Europe has projected Moly shortages to continue through the fourth week of November. As has been the case throughout this period of reactor schedule changes, Lantheus remains fully capable of supplying our regular standing orders thanks to our globally diversified and balanced Moly supply chain. Further, we continue to work closely with our suppliers to increase production capacity for additional orders from our customers.
Regarding our Xenon-133, which is sourced solely from European reactors, we will not have a production run this Wednesday, November 23 and next Wednesday, November 30. We will return to normal production on Wednesday, December 7. Given the successful restart of LVR15, we have high confidence in this return date.
Please stay in touch with your Lantheus Nuclear Medicine Sales Representative for the latest information on additional TechneLite and Xenon‐133 availability. We believe our actions may alleviate some of the impact of this disruption to you, your operations, and the patients we all serve.
As this situation evolves, we will continue to provide additional information. Should you have any questions or need additional information, please contact your Lantheus Nuclear Medicine Sales Representative or Lantheus Customer Service at 1‐800‐299‐3431.
Lantheus Circles the Globe to Bring TechneLite® to You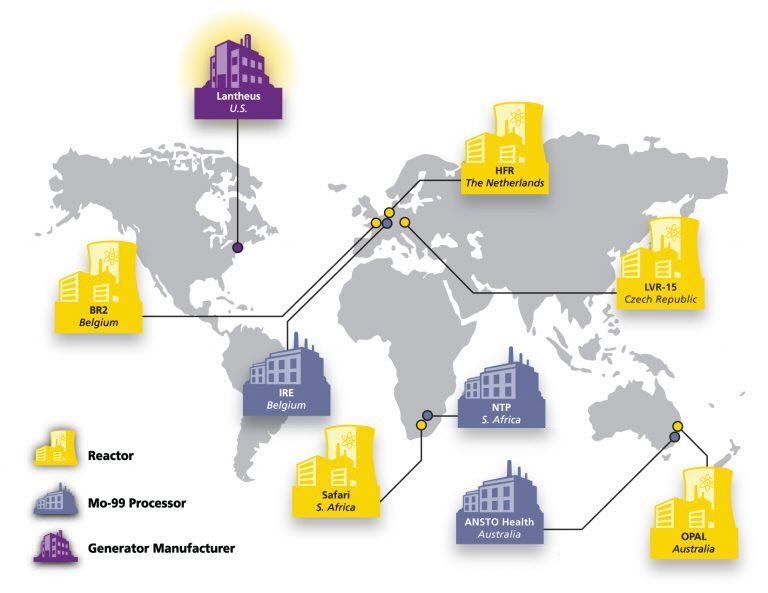
As a leader in the radiopharmaceutical business, Lantheus has developed a world class, globally diversified and balanced Molybdenum-99 (Mo-99) supply chain for the procurement of Mo-99. The company presently receives Mo-99 from three of the four major Mo-99 processors and five of the six associated producing reactors.


